What Are Biosimilars? A Simple, Patient-Friendly Guide

When you hear the word "biosimilar," it might sound confusing-like a mix of "biological" and "similar." But here’s the simple truth: biosimilars are medicines that work almost exactly like a well-known biologic drug, but they cost less. They’re not experimental. They’re not guesswork. They’re science-backed, FDA-approved, and used by millions around the world.
What’s the difference between a biosimilar and a generic drug?
Most people know what generics are. If you’ve ever filled a prescription for a cheaper version of a pill like ibuprofen or metformin, you’ve used a generic. Generics are exact chemical copies of brand-name drugs. They have the same active ingredient, the same dose, the same effect. Simple. Biosimilars are different. They’re not exact copies. Why? Because they’re not made from chemicals. They’re made from living cells-like tiny factories inside a lab that produce complex proteins. Think of it like baking a cake. A generic is like using the exact same recipe, same flour, same sugar, same eggs. A biosimilar is like using a different oven, a different brand of flour, and a slightly different mixing method-but the cake still tastes the same, rises the same, and feeds the same number of people. The FDA says biosimilars must be "highly similar" to the original biologic. That means every important part-the shape, the structure, how it works in your body-must match. Any tiny differences? They have to be so small they don’t change how safe or effective the medicine is.How are biosimilars made?
Biologic drugs-like Humira, Enbrel, or Herceptin-are made using living organisms. Usually, scientists take a human gene, put it into a cell (often from a hamster or insect), and let that cell produce the protein medicine. It’s not like mixing chemicals in a beaker. It’s more like growing a plant in a greenhouse. A biosimilar follows the same idea. Another company takes the same gene, uses their own cells, their own fermentation tanks, their own purification steps. The result? A medicine that looks, acts, and works just like the original. But because living systems are never perfectly identical, you can’t get an exact copy. That’s why they’re called "biosimilars," not "copies."Are biosimilars safe?
Yes. The FDA doesn’t approve a biosimilar unless it’s been tested in labs, animals, and sometimes hundreds of patients. For example, the biosimilar for the cancer drug Herceptin went through a study with over 500 patients. No safety issues. No drop in effectiveness. In Europe, biosimilars have been used for over 15 years. Millions of people have taken them. No unexpected side effects. No long-term problems. The same is true in the U.S. Since the first one, Zarxio, was approved in 2015, dozens more have followed-each with the same strict review process. The American Cancer Society says it clearly: "A biosimilar behaves in much the same way as its brand name biologic. There are no meaningful differences between how the two medicines work."What conditions do biosimilars treat?
Biosimilars are used for serious, long-term illnesses where biologics are the best option:- Rheumatoid arthritis and other autoimmune diseases
- Crohn’s disease and ulcerative colitis
- Psoriasis and eczema
- Diabetes (biosimilar insulins)
- Certain cancers (like breast cancer, colon cancer, lymphoma)
- Macular degeneration (a leading cause of vision loss)
- Chronic kidney disease

Do biosimilars cost less?
Yes-and that’s the whole point. Biologics can cost $10,000 to $20,000 a year. Biosimilars usually cost 15% to 30% less. That’s not just savings for you-it’s savings for the whole healthcare system. Some insurance plans now require you to try a biosimilar first before they’ll pay for the original. That’s not because they think it’s worse. It’s because it’s just as good, and cheaper. There’s even a new category called "interchangeable" biosimilars. These can be swapped for the original drug without your doctor having to write a new prescription. The first one, Semglee (a biosimilar to Lantus insulin), was approved in 2021. More are coming.How do I know if I’m getting a biosimilar?
Your medicine will have a different name. The original biologic might be called "adalimumab." The biosimilar might be called "adalimumab-adsd" or "adalimumab-abda." That four-letter ending is there so doctors and pharmacists can tell them apart. It’s not random-it’s part of a global naming system. You’ll also see it on your prescription label. If you’re unsure, ask your pharmacist. They’re trained to know the difference.Can I switch from a biologic to a biosimilar?
Yes. Studies show switching is safe. In fact, many patients switch without even noticing a difference. The Arthritis Foundation and other patient groups say switching doesn’t increase side effects or reduce effectiveness. Your doctor will decide if switching is right for you. If you’ve been stable on your current drug, they might keep you on it. If cost is a problem, or if your insurance pushes for a biosimilar, switching is a smart option.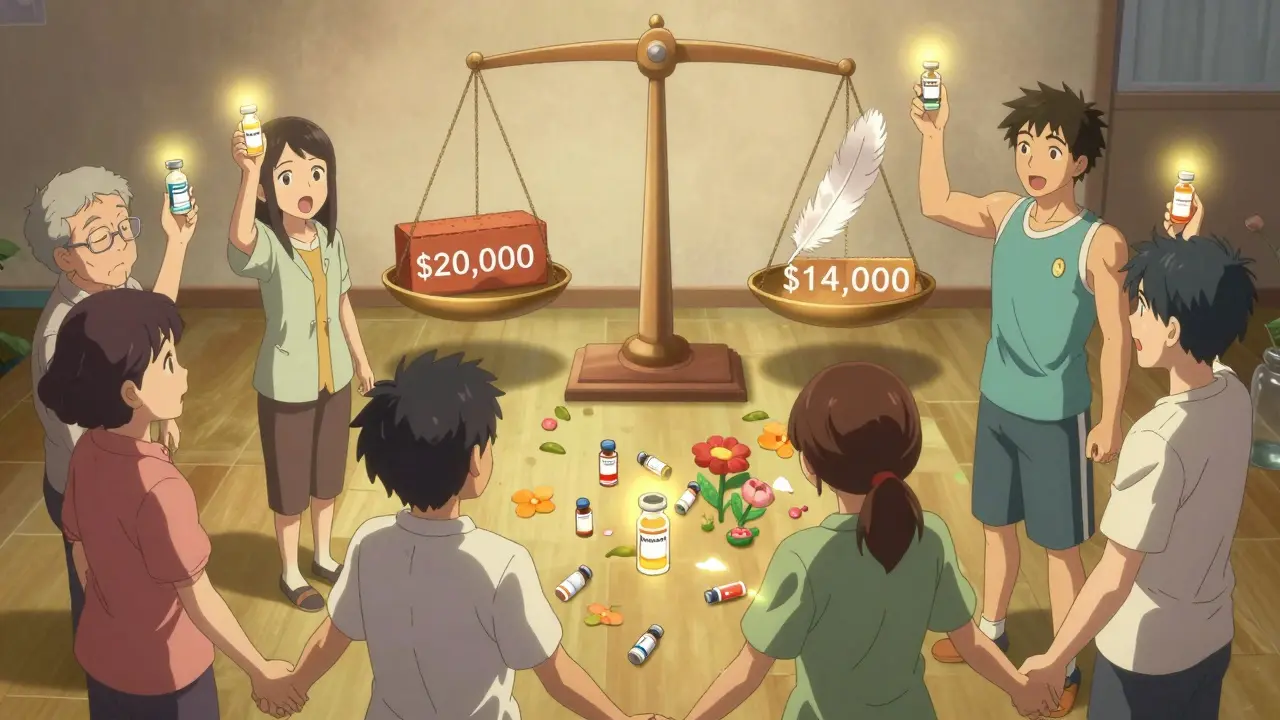
What about side effects?
Biosimilars have the same side effects as the original biologic. That’s required by law. If the original causes fatigue, joint pain, or an increased risk of infection, so will the biosimilar. But here’s the good news: because biosimilars are closely monitored after they’re approved, any new safety issue shows up fast. The FDA tracks every single one. If something odd happens, they find it quickly.Why aren’t biosimilars used more in the U.S.?
In Europe, about 25% of biologic prescriptions are for biosimilars. In the U.S., it’s still around 10%. Why the gap? Some doctors are still learning about them. Some patients worry they’re "second-best." And some drug companies fight hard to protect their original brands-through legal battles, marketing, and confusing messaging. But the trend is shifting. More biosimilars are coming. More doctors are prescribing them. More patients are asking for them. And with over $50 billion in projected healthcare savings by 2026, the system is moving toward them.What should I do if I’m prescribed a biosimilar?
Ask three simple questions:- Is this a biosimilar? (Check the name on the label.)
- How does it compare to the original drug I’ve been taking?
- Will this change how I feel or what side effects I get?
Are biosimilars the same as generic drugs?
No. Generic drugs are exact chemical copies of small-molecule pills, like aspirin or statins. Biosimilars are highly similar versions of complex biologic drugs made from living cells. They can’t be exact copies because the molecules are too big and complex. But they work the same way and have the same safety profile.
Are biosimilars safe for long-term use?
Yes. Biosimilars are approved only after years of testing-analytical studies, animal trials, and clinical trials in humans. In Europe, they’ve been used safely for over 15 years. In the U.S., since 2015, thousands of patients have used them without new or unexpected safety risks.
Can I switch from my current biologic to a biosimilar?
Yes. Multiple studies show switching is safe and effective. Many patients switch without noticing any difference in how they feel. Your doctor will help decide if switching is right for you, especially if cost or insurance coverage is a concern.
Do biosimilars have different side effects than the original?
No. By law, biosimilars must have the same potential side effects as the original biologic. If the original causes fatigue, infection risk, or injection-site reactions, so will the biosimilar. Any differences would mean it didn’t pass FDA approval.
Why do biosimilars have strange names with four-letter endings?
The four-letter suffix helps doctors, pharmacists, and patients tell biosimilars apart from the original drug and from each other. It’s part of a global system designed to track safety and avoid confusion. For example, infliximab is the original; infliximab-dyyb is a biosimilar. It doesn’t mean it’s different in quality-it just helps with tracking.
Are biosimilars covered by insurance?
Yes. Most insurance plans cover biosimilars, and many require you to try one first because they’re cheaper. Even if your plan doesn’t cover the original biologic, it’s likely to cover the biosimilar. Always check with your insurer or pharmacist.


Daniel Dover
February 16, 2026 AT 04:49Biosimilars are a game changer. No more paying $20k a year for the same medicine. Science doesn't care about brand names. It cares about results. And the results? Same efficacy, same safety, way cheaper. Simple.
Charlotte Dacre
February 17, 2026 AT 13:03Oh great, another corporate miracle wrapped in FDA paperwork. You mean to tell me after decades of drug companies charging $10,000 a dose, suddenly they’re okay with ‘similar’? Funny how ‘similar’ only becomes acceptable when it costs 30% less. Thanks for the PR spin, but I’ll stick to my original. Just kidding-I switched last year. Saved $8K. And nope, didn’t turn into a pumpkin.
Chiruvella Pardha Krishna
February 17, 2026 AT 17:26The philosophical underpinnings of biosimilarity reveal a deeper truth about human attempts to replicate nature. Living systems are not algorithms. They are emergent phenomena. To claim equivalence is to impose a reductionist framework on a biological symphony. Yet, paradoxically, the clinical outcomes suggest harmony despite asymmetry. The body does not discern the origin of the protein-it only responds to its function. Thus, in the silence of physiology, we find the truest form of equivalence: not in structure, but in effect.
Mandeep Singh
February 19, 2026 AT 03:50Let me tell you something. People are scared of biosimilars because they don’t understand biology. They think if it’s not the exact same molecule, it’s a knockoff. That’s not science-that’s fear. I’ve been on biosimilars for five years. My RA is controlled. My insurance paid less. My doctor didn’t lie. The FDA didn’t cut corners. And yet, people still think it’s some kind of scam. The real scam is paying full price for a drug that’s been replicated with the same clinical trials, the same safety data, the same outcomes. Stop being lazy. Ask for the biosimilar. Your wallet and your body will thank you.
Kaye Alcaraz
February 20, 2026 AT 11:07For anyone reading this and wondering whether to switch-do it. The data is solid. The safety profile is identical. The cost savings are real. Millions have done it. No hidden risks. No surprise side effects. If your doctor recommends it, trust the evidence. You’re not downgrading-you’re upgrading to a smarter healthcare system.
Sarah Barrett
February 22, 2026 AT 00:09It’s wild how we treat medicine like a luxury brand. ‘Oh, I need the original, it’s the *experience*.’ But a biosimilar isn’t a knockoff handbag-it’s a different factory making the exact same engine. The name changes, the price drops, the outcomes? Still perfect. I switched from Humira to its biosimilar. Didn’t feel a thing. Except maybe a little lighter in my wallet. And honestly? That’s the real miracle.
Erica Banatao Darilag
February 22, 2026 AT 20:05i just want to say that i switched to a biosimilar last year and it was the best decison i ever made. my doc explained evrything and i felt so much less stress about cost. also the injection was the same. no diff. just less money spent. thank you for making this info clear.
Kapil Verma
February 22, 2026 AT 22:10Why are Americans so afraid of innovation? In India, we’ve been using biosimilars for over a decade. We don’t worship Western brands. We use what works. Biosimilars are not inferior-they are intelligent adaptations. If you’re still clinging to the original because of some brand loyalty, you’re not protecting your health-you’re protecting corporate profits. Shame on you for letting marketing dictate your medicine.
Michael Page
February 24, 2026 AT 18:48There’s an irony in how we equate familiarity with safety. We trust the original because we’ve heard its name. But the biosimilar carries the same weight of evidence-just without the advertising budget. The body doesn’t read pharmaceutical brochures. It responds to molecular shape, receptor binding, pharmacokinetics. The rest is noise.
Joe Grushkin
February 25, 2026 AT 07:46Let’s be real-biosimilars are just Big Pharma’s way of extending monopolies under a new name. They don’t want you to know that the original drug’s patent expired. So they slap on a four-letter suffix and call it ‘innovation.’ Meanwhile, the same lab that made Humira is probably making the biosimilar. It’s a shell game. And you’re the sucker paying less because you think you’re being smart. You’re not. You’re being manipulated.