Humira Biosimilar Coverage: What You Need to Know About Cost, Access, and Alternatives
When you’re managing a chronic condition like rheumatoid arthritis or Crohn’s disease, Humira, a brand-name biologic drug used to suppress the immune system in autoimmune diseases. Also known as adalimumab, it’s been a lifeline for millions—but its price tag has pushed many to look for alternatives. Enter Humira biosimilars, medications that are nearly identical to Humira in structure and function but are sold at a fraction of the cost. These aren’t generics—they’re complex biological products made from living cells, and getting them covered by insurance isn’t always straightforward.
Biosimilar drugs, including adalimumab biosimilars like Amjevita, Cyltezo, and Hyrimoz. are approved by the FDA after proving they work just like Humira with no meaningful difference in safety or effectiveness. Yet coverage still varies wildly. Some insurers automatically switch you to a biosimilar to save money. Others make you jump through hoops—prior authorization, step therapy, even appeals. And some plans still favor the original brand, even when the biosimilar is cheaper. Why? Because drug manufacturers pay rebates to insurers to lock in brand loyalty. It’s not about what works best—it’s about what’s most profitable.
If you’re on Humira and your plan starts pushing a biosimilar, don’t panic. Ask your doctor to write a letter of medical necessity if you’ve had side effects or poor response to other treatments. Check your formulary—many plans list biosimilars in Tier 2, meaning lower copays than Humira’s Tier 3 or 4. And if your insurer denies coverage, know that many biosimilar makers offer patient assistance programs that can slash out-of-pocket costs to under $5 a month. You’re not alone in this fight. Thousands have successfully switched, and the data shows biosimilars work just as well.
What you’ll find below are real, practical guides that connect the dots between insurance rules, drug safety, and patient rights. From how generic drug competition drives down prices to why pharmacogenetic testing, a tool that analyzes how your genes affect drug response. might help predict your reaction to biologics, these posts give you the tools to navigate the system. You’ll also learn how medication safety, the practice of avoiding harmful drug interactions and dosing errors. applies to biosimilars, why generational attitudes, how different age groups view generic and biosimilar drugs. matter when switching treatments, and how to spot red flags in online pharmacy claims. This isn’t theory—it’s what people are actually dealing with right now. Let’s get you the info you need to make the right call.
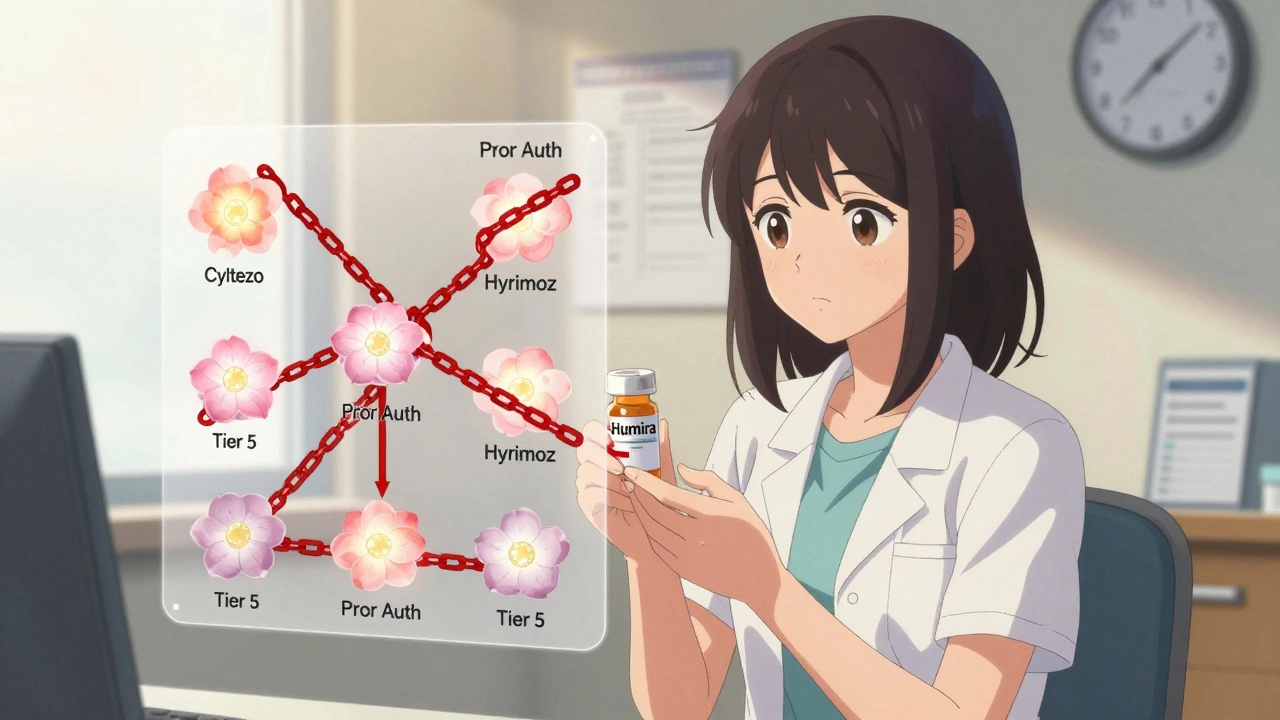
Insurance Coverage of Biosimilars: How Prior Authorization and Tier Placement Block Savings
Despite FDA approval of over 70 biosimilars, most insurance plans still treat them the same as expensive biologics like Humira-same tier, same prior authorization. This blocks savings and delays patient access.
VIEW MORE