Anastrozole in Endocrine Therapy: Benefits, Risks, and Patient Guide

You want the truth about hormone therapy-not sugar-coated promises. Here it is: for the right person, Anastrozole can cut the risk of breast cancer coming back by a meaningful margin. But it trades hot flashes and joint aches for fewer clots and uterine problems. You’ll likely take it for years, and the small daily choices you make will decide how well you tolerate it. No miracle claims-just what it does, who it helps, and how to manage the bumps.
- TL;DR: Anastrozole (an aromatase inhibitor) lowers estrogen and reduces recurrence of hormone receptor-positive breast cancer, especially in postmenopausal patients.
- Benefits: In trials like ATAC and EBCTCG meta-analyses, AIs lower recurrence more than tamoxifen; typical absolute benefit is a few percentage points, higher with node-positive disease.
- Trade-offs: More bone loss and joint pain than tamoxifen; fewer blood clots and no uterine cancer risk. Heart risks are slightly higher with AIs versus tamoxifen.
- Best fit: Postmenopausal HR+ early-stage breast cancer; premenopausal only with ovarian suppression. Extended therapy (7-10 years total) helps higher-risk cases.
- Make it tolerable: Baseline DEXA, vitamin D, strength training, pain tactics for joints, and a plan with your oncologist. Don’t quit without trying fixes.
How Anastrozole Fits Into Endocrine Therapy: Benefits, Who It Helps, and the Real Numbers
Anastrozole is a once-daily aromatase inhibitor (AI). It blocks the enzyme that makes estrogen outside the ovaries. Less estrogen means fewer signals for hormone receptor-positive (HR+) breast cancer cells to grow. It’s a core drug in adjuvant therapy after surgery (with or without chemo and radiation) and sometimes in metastatic settings.
Who is it usually for? Postmenopausal women with HR+ breast cancer. It can also work in premenopausal patients, but only if the ovaries are suppressed (with drugs like goserelin or with surgery). It’s not a fit for triple-negative cancers and doesn’t replace chemo when chemo is indicated; it complements it by tackling hormone-driven risk long term.
What do the big studies say? The ATAC trial (Lancet, long-term follow-up) compared anastrozole with tamoxifen in early HR+ breast cancer. Anastrozole improved time to recurrence and cut new cancers in the other breast. The absolute gains depend on your baseline risk-think a few percentage points at 5-10 years-but those points matter when we’re talking about staying cancer-free. The Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analyses showed that across many trials, AIs reduce recurrence more than tamoxifen and lower breast cancer mortality modestly over the long run. People with lymph node involvement usually see the biggest absolute benefit.
How much benefit, roughly? As a rule of thumb: if your risk of recurrence over 10 years is 20%, an AI instead of tamoxifen might drop that by a few percent. If your baseline risk is 10%, the absolute drop is smaller. That’s why your pathology (tumor size, nodes, grade), genomic tests (like Oncotype DX if used), and age guide the call.
Extended therapy-does it help? Trials like MA.17R (NEJM) tested five extra years of an AI after five years of prior endocrine therapy. Result: better disease-free survival and fewer contralateral breast cancers, but more fractures and no clear overall survival gain for everyone. NSABP B-42 and IDEAL found similar patterns. Bottom line: people at higher risk (positive nodes, large tumors, high grade) tend to benefit from 7-10 total years. For lower-risk cases, five years may be enough. Personalized risk, not a blanket rule.
How about metastatic disease? Anastrozole is often part of first-line hormone therapy for HR+ metastatic breast cancer. It’s commonly paired with targeted drugs like CDK4/6 inhibitors to delay progression. Different ballgame, but the logic is the same-starve the cancer of estrogen signaling.
What if I’m premenopausal? On its own, anastrozole won’t work if your ovaries are active. But with ovarian suppression (injections every 4 weeks or surgery), an AI can outperform tamoxifen in higher-risk premenopausal patients. The SOFT and TEXT trials showed that adding ovarian suppression to endocrine therapy improved disease-free survival, especially in women who needed chemotherapy. It’s a bigger commitment-more side effects to manage-but it’s powerful for the right person.
So, the promise is real. The proof is long-standing. And the size of your benefit is tied to your risk-more risk, more to gain.
Risks, Side Effects, and How to Lower Them (Bone, Heart, Joints, Sexual Health)
Every drug brings a trade-off. With anastrozole, bone health and joint pain are the big ones. Tamoxifen has clots and uterine cancer on its list; AIs swap those out for musculoskeletal and bone issues. Know what to expect and how to blunt it.
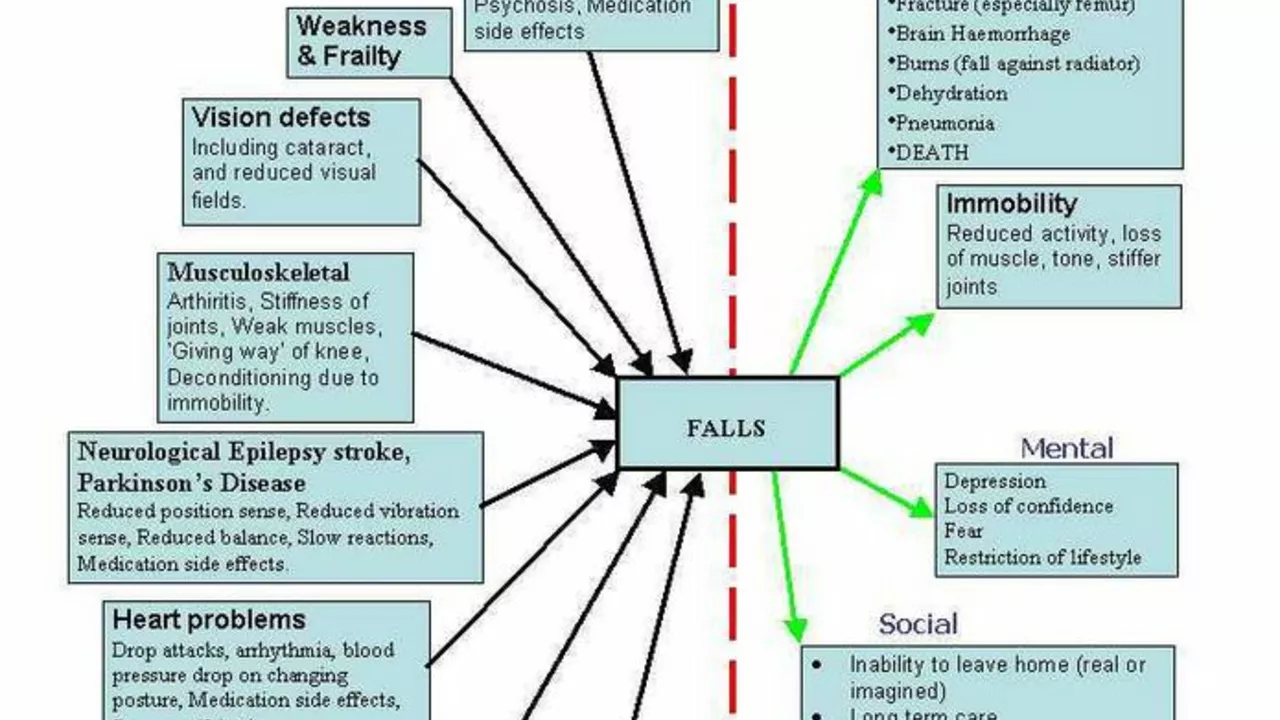
Bone loss and fractures: AIs speed up bone turnover. In ATAC, fractures were more common on anastrozole than tamoxifen. Plan ahead. Get a baseline DEXA scan, repeat every 1-2 years, and start bone measures early-don’t wait for a fracture. Calcium (diet first, supplement if needed) and vitamin D keep the basics covered, but they’re not enough alone if your bone density is slipping.
What works to protect bones? Weight-bearing and resistance training, regular walking, and avoiding smoking make a real difference. If your DEXA shows osteopenia or osteoporosis, your team may add a bisphosphonate (like zoledronic acid or oral alendronate) or denosumab. There’s a bonus here: an EBCTCG analysis on adjuvant bisphosphonates showed fewer bone metastases and better breast cancer outcomes in postmenopausal women. So bone protection can carry anti-cancer upside.
Joint aches and stiffness: This is the side effect that makes people think about quitting. Morning stiffness, hands, knees, hips-it’s common. The plan? Move daily. Short, frequent activity beats weekend warrior workouts. Try anti-inflammatory measures: topical NSAID gel on painful joints, warm showers on waking, stretching. If it’s bad, ask about switching within the AI class (anastrozole to letrozole or exemestane). Sometimes a simple switch helps. Acupuncture has support in randomized trials for AI-related arthralgia. If you sit a lot for work, set a timer to stand and walk every hour.
Hot flashes and sweats: AIs can still cause them, though often less than tamoxifen. Dress in layers, use a fan at night, and avoid triggers like alcohol and spicy food close to bedtime. Nonhormonal options such as venlafaxine, gabapentin, or clonidine can help. If you’re on tamoxifen now and switching later, remember: some antidepressants block tamoxifen’s activation; that’s less of a worry with AIs.
Vaginal dryness and sexual pain: Low estrogen affects tissue moisture and elasticity. Water- or silicone-based lubricants help. Vaginal moisturizers used several times a week help maintain tissue health. Pelvic floor physical therapy can reduce pain and improve function. Nonhormonal tactics come first. If nothing works, talk to your oncologist about ultra-low-dose vaginal estrogen. Guidelines allow it after shared decision-making, with extra caution in AI users.
Heart and cholesterol: Compared with tamoxifen, AIs don’t have the same lipid-friendly effects, and some studies show a slight uptick in cardiovascular events with AIs. If you have heart disease, high blood pressure, diabetes, or a smoking history, double down on risk control. Get lipids checked. Focus on Mediterranean-style eating, regular cardio, and blood pressure control. If your LDL climbs, a statin may make sense-decide with your doctor.
Mood, sleep, and cognition: Fatigue, low mood, and brain fog can show up. Routine helps-regular sleep hours, daylight exposure, and gentle exercise most days. If you’re stuck, ask for help sooner rather than later. Cognitive symptoms often improve over months; either way, there are workarounds (lists, timers, chunking tasks).
Clots and uterine cancer: This is where AIs shine compared with tamoxifen. AIs do not increase uterine cancer risk and carry a much lower clot risk. If you’ve had a clot before, this may lean the choice toward an AI.
Allergies and rare issues: Serious allergic reactions are uncommon. Liver enzyme bumps can happen; routine bloodwork can catch it. Report yellowing eyes, severe itching, or dark urine.
| Drug | Class | Best for | Key benefits | Main risks | Notes |
|---|---|---|---|---|---|
| Anastrozole | Aromatase inhibitor | Postmenopausal HR+; premenopausal with ovarian suppression | Lower recurrence vs tamoxifen during treatment | Bone loss, fractures, joint pain; possible lipid rise | Daily pill; consider bone agents if osteopenia/osteoporosis |
| Letrozole | Aromatase inhibitor | Similar to anastrozole; often used in extended therapy | Strong suppression of estrogen; data for extension | Similar bone/joint issues | Switching within AI class can help joint pain |
| Exemestane | Aromatase inhibitor (steroidal) | Option if joint pain on other AIs; with ovarian suppression in high-risk premenopausal | Alternative when others not tolerated | Bone loss, joint pain | Different structure; sometimes easier to tolerate |
| Tamoxifen | Selective estrogen receptor modulator | Premenopausal HR+ without ovarian suppression; bone protection in postmenopausal | Lower fractures; helpful if bone density is low | Clots, uterine cancer risk, hot flashes | Interactions with some antidepressants (CYP2D6) |

How to Take Anastrozole Day to Day: Dosing, Duration, Monitoring, and What to Do When Things Go Sideways
Dose: 1 mg by mouth once daily. Food or no food-it doesn’t matter. Take it the same time each day to make it a habit. If you miss a dose and remember within the day, take it. If it’s close to the next dose, skip the missed one. Don’t double up.
When to start: Often after finishing radiation, or as advised by your oncologist if you had chemo. Some start sooner. A few weeks doesn’t change long-term outcomes; the key is steady use over years.
How long: Most people do 5 years. If risk is higher, you and your team may aim for 7-10 years total endocrine therapy (sometimes switching between drugs). If side effects are rough, you can try breaks or switches. Short breaks don’t erase the benefit you’ve built, but aim for the plan you agreed on.
Premenopausal path: If you’re premenopausal, anastrozole must be paired with ovarian suppression. That means monthly shots (or surgery) and the same day-to-day steps, plus extra attention to bone and mood changes. Stopping suppression while staying on anastrozole makes the AI ineffective-flag any missed shots right away.
What labs and scans: Get a baseline DEXA scan before or soon after starting. Then repeat every 12-24 months, faster if you’re losing bone. Lipids at baseline and at 3-12 months depending on your history. Basic blood tests a few times a year. If you have joint pain, you don’t need routine imaging, but you do need a plan.
What to avoid: Don’t take estrogen-containing hormone therapy. Herbal products that act like estrogen (black cohosh, red clover) are a gray zone-discuss first. Grapefruit doesn’t have a major interaction signal here, but keep supplements simple and tell your team what you use. Alcohol in moderation is fine unless your doctor says otherwise.
Pregnancy and birth control: AIs can harm a fetus. If you can become pregnant, use effective birth control even if periods stop. Nonhormonal options are preferred; copper IUDs are a common choice. Ask your team to coordinate a safe plan.
Side-effect action plan, step by step:
- Week 1-2: Set your routine. Daily walk (15-30 minutes). Start vitamin D if low. Log symptoms on your phone. Hydrate.
- Week 3-4: Add two short resistance sessions per week (bodyweight or bands). Try a nighttime fan or cooling bedding if flashes hit.
- Month 2-3: If joints hurt, add morning stretches and a warm shower. Consider topical NSAID gel. Ask about physical therapy if stiffness limits you.
- Month 3-4: If pain is 6/10 or you’re thinking about quitting, talk to your oncologist. Consider switching AIs or a brief break to reset. Try acupuncture if available.
- Any time: If mood tanks, sleep is awful, or sex is painful, speak up. There are fixes, and you don’t have to tough it out.
Monitoring schedule you can copy:
- Baseline: DEXA, vitamin D, lipids, blood pressure, weight, exercise plan.
- 3 months: Symptom check; adjust pain plan; check lipids if high risk.
- 6-12 months: Repeat DEXA if osteopenia/osteoporosis; otherwise at 12-24 months.
- Yearly: Heart risk review, lifestyle tune-up, and a revisit of the benefit-risk balance.
When to call now: Sudden shortness of breath or chest pain (urgent). Severe, new back pain that doesn’t ease-especially if you had low bone density (possible vertebral fracture). Yellowing eyes or dark urine (liver). Any vaginal bleeding after menopause needs evaluation, though AIs don’t raise uterine cancer risk.
Smart Decisions and FAQs: When to Choose It, How It Compares, and What to Ask Your Oncologist
Here’s a simple way to think through the decision:
- If you’re postmenopausal with HR+ early breast cancer: an AI like anastrozole is usually first choice because it lowers recurrence more than tamoxifen while you’re on it.
- If you’re premenopausal: either tamoxifen or AI plus ovarian suppression. If your risk is higher (for example, lymph nodes positive), AI plus suppression often gives more protection.
- If your bones are fragile or you’ve had fractures: either plan stronger bone protection with an AI or consider starting with tamoxifen and reevaluate later.
- If you’ve had a blood clot or uterine issues: an AI may be safer than tamoxifen.
- If joint pain becomes a barrier: try switching AIs or a structured symptom plan before giving up on endocrine therapy.
Key questions to ask your oncologist:
- What’s my estimated absolute benefit from an AI versus tamoxifen given my stage and nodes?
- Do I need 5 years or closer to 7-10 years of total therapy?
- What’s our bone plan-DEXA timing, vitamin D dose, and do I qualify for a bisphosphonate or denosumab?
- If joint pain hits, what’s the step-by-step plan? Can I switch within the AI class?
- Are there reasons I should start with tamoxifen instead?
- How will we watch my heart risk and cholesterol?
Common myths, quickly cleared:
- “If I miss a few days, the whole plan is ruined.” Not true. Get back on track. Consistency matters over months and years, not a single day.
- “All joint pain means I need to stop.” No. Most people can improve symptoms with activity, topical meds, and sometimes a switch.
- “I can’t exercise on this.” You can, and it usually helps pain, energy, and sleep.
- “Vaginal estrogen is never allowed.” Nonhormonal first. If that fails, ultra-low-dose vaginal estrogen can be considered with careful discussion, especially on AIs.
Mini-FAQ
- Does anastrozole work if I’m still getting periods? No-unless your ovaries are suppressed. Periods can be irregular on chemo; your team may check hormones to confirm menopausal status.
- Is generic anastrozole as good as brand? Yes. Regulated generics match the active ingredient and effectiveness.
- Can I take supplements like turmeric or glucosamine for joint pain? Usually fine, but tell your team. Keep doses reasonable and watch for interactions if you’re on other meds.
- How soon will I feel side effects? Hot flashes may show up in weeks, joint aches over 1-3 months, bone effects take longer. Report changes early.
- Can I stop early if I’m cured? Endocrine therapy reduces risk while you take it and for some time after, but stopping early can give up protection. Always talk before stopping.
- How will we know it’s working? You won’t “feel” it working. The benefit shows up in long-term stats-fewer recurrences over years.
Quick checklists you can save:
Starter checklist (before or soon after day 1)
- Confirm HR+ status and menopausal status (lab if unclear).
- Decide AI vs tamoxifen based on your risk and preferences.
- Order baseline DEXA; check vitamin D and lipids.
- Set daily pill time and a simple symptom log.
- Start a walking plan; line up resistance bands or gym access.
- Discuss bone-protective therapy if osteopenia/osteoporosis is present.
Side-effect toolbox
- Joint pain: daily movement, morning heat, topical NSAID, PT, acupuncture, consider AI switch.
- Hot flashes: layers, cool bedroom, avoid late alcohol/spicy food; venlafaxine/gabapentin if needed.
- Vaginal dryness: lubricants during sex, moisturizers twice weekly; pelvic floor PT; consider ultra-low-dose vaginal estrogen if nonhormonal options fail.
- Bone health: DEXA on schedule, vitamin D repletion, strength training, bisphosphonate/denosumab when indicated.
- Mood/sleep: consistent bedtime, sunlight exposure, light exercise; seek help early if symptoms persist.
Decision snapshots
- Low-risk, node-negative, postmenopausal, osteopenia: Tamoxifen may be reasonable; or AI with strong bone plan. Personal preference matters.
- High-risk, node-positive: AI-based plan is favored; consider extended therapy beyond 5 years.
- Premenopausal, chemo given, high risk: AI plus ovarian suppression is often the strongest endocrine plan.
Sources that guide this advice: ATAC trial (Lancet), EBCTCG meta-analyses (Lancet), MA.17R (New England Journal of Medicine), NSABP B-42 and IDEAL (Journal of Clinical Oncology), SOFT/TEXT (New England Journal of Medicine). If you want the exact papers, your oncology team can pull them up, but the gist here matches the consensus used in clinics today.
Next steps and troubleshooting
- If you haven’t started therapy: schedule a DEXA, write down your top three concerns, and bring them to your next visit.
- If you’re on it and hurting: don’t white-knuckle it. Ask about a structured joint-pain plan or a switch. Most people can keep going with adjustments.
- If you finished 5 years: ask whether your risk profile points to extended therapy. A short talk and a DEXA can clarify the path.
- If life is busy and you forget pills: set a phone alarm, pair the pill with a daily habit (toothbrushing), and keep a spare in your bag.
- If you’re worried about bones: push for a clear bone plan today-DEXA timing, vitamin D target, and whether you qualify for bone medication.
You don’t have to love every minute of endocrine therapy to benefit from it. You do need a plan, a way to measure what matters, and a team that tweaks things with you. That combination-good science, steady habits, and honest feedback-gets most people through the years that count.


Tim Giles
August 28, 2025 AT 21:48An important aspect of anastrozole therapy is the systematic assessment of bone mineral density, because aromatase inhibition accelerates bone turnover and can precipitate osteopenia or frank osteoporosis if left unchecked. Baseline dual‑energy X‑ray absorptiometry (DEXA) should be obtained before initiating treatment, and repeat scans at intervals of 12 to 24 months allow clinicians to detect early loss and intervene promptly. In addition to calcium‑rich dietary sources, maintaining serum 25‑hydroxy vitamin D concentrations in the optimal range (generally above 30 ng/mL) is essential for supporting calcium absorption and mitigating skeletal deterioration. When DEXA results reveal T‑scores in the osteopenic range, the addition of a bisphosphonate such as alendronate or an intravenous agent like zoledronic acid is often warranted, and these agents have been shown to reduce both fracture risk and, in some analyses, breast cancer recurrence. For patients with established osteoporosis, denosumab offers an alternative mechanism of action by inhibiting RANK‑L, thereby decreasing osteoclast activity. The selection of a bone‑protective strategy should be individualized, taking into account renal function, patient preference, and potential drug interactions with other supportive medications. Regular weight‑bearing and resistance exercises further complement pharmacologic measures, providing mechanical stimulus that promotes bone remodeling and muscle strength. Ultimately, a proactive, multidisciplinary approach to bone health enables patients to remain on anastrozole for the intended duration while minimizing the likelihood of fracture‑related morbidity.
Peter Jones
August 29, 2025 AT 01:50I appreciate the comprehensive outline of monitoring protocols, and incorporating a simple symptom diary can make it easier for patients to communicate joint discomfort, hot flashes, or mood changes during clinic visits.
Gerard Parker
August 29, 2025 AT 22:40Listen, the evidence from MA.17R and subsequent meta‑analyses makes it unequivocally clear that extending an aromatase inhibitor beyond five years yields a measurable disease‑free survival advantage in high‑risk patients, and there is no legitimate excuse to dismiss this benefit as marginal; the incremental absolute reduction in recurrence, while modest in numeric terms, translates into dozens of lives spared from metastatic disease when applied across a population. Moreover, the notion that bone toxicity should automatically preclude extended therapy is a misconception, because concurrent administration of bisphosphonates or denosumab effectively neutralizes the fracture risk without compromising oncologic efficacy. Clinicians must therefore adopt a dual‑targeted strategy: aggressive oncologic suppression through continuous AI exposure paired with vigilant skeletal protection, rather than defaulting to a premature cessation that undermines the long‑term cure potential. This approach aligns with guideline recommendations and reflects a commitment to maximizing the therapeutic index for each individual.
Thomas Burke
August 30, 2025 AT 01:26Switching to letrozole can sometimes ease arthralgia.
Debbie Frapp
August 31, 2025 AT 02:26First, let’s celebrate how many actionable steps we can pull from this guide, because knowledge is the first weapon against treatment‑related adversity. Understanding that anastrozole suppresses estrogen synthesis is the foundation, and recognizing that this suppression is both a blessing for tumor control and a curse for bone health sets the stage for proactive management. The next step is to schedule that baseline DEXA scan as soon as the prescription is written, because early detection of low bone density allows us to intervene before a fracture occurs. Vitamin D supplementation should be personalized; many patients benefit from an initial loading dose followed by a maintenance regimen that keeps serum levels in the optimal range, and this can be verified with a simple blood test. Calcium intake through dairy, fortified plant milks, or supplements should aim for the recommended 1,200 mg per day, and spreading the dose throughout the day improves absorption. Resistance training, such as body‑weight squats, lunges, and resistance‑band exercises, performed three times weekly, provides the mechanical stimulus that drives bone formation while simultaneously reducing joint stiffness. If arthralgia becomes intolerable, topical NSAID gels can be applied locally, and a brief course of oral NSAIDs may be prescribed, but always watch for gastrointestinal side effects. Acupuncture has a growing evidence base for alleviating AI‑induced joint pain, and many clinics now offer it as part of supportive care packages. Should pain persist despite these measures, a strategic switch to another aromatase inhibitor-letrozole or exemestane-can sometimes reset the pain pathways and provide relief. For patients who develop osteopenia or osteoporosis, initiating a bisphosphonate like alendronate or an intravenous agent such as zoledronic acid not only protects bone but may also carry a modest anti‑cancer benefit, as demonstrated in large adjuvant trials. Denosumab is an alternative for those with contraindications to bisphosphonates, and its subcutaneous administration every six months offers convenience. Regular follow‑up visits should include not just a review of side effects but also a structured assessment of mood, sleep, and cognitive function, because fatigue and brain fog can be as debilitating as physical symptoms. Non‑pharmacologic strategies-consistent sleep hygiene, daytime sunlight exposure, and mindfulness practices-can mitigate these issues, and when they fail, low‑dose antidepressants like venlafaxine are evidence‑based options for hot flashes and mood disturbances. Sexual health should not be ignored; water‑based lubricants and weekly vaginal moisturizers maintain tissue health, and pelvic floor physical therapy can improve comfort during intercourse. If these measures are insufficient, a carefully monitored trial of ultra‑low‑dose vaginal estrogen may be considered after detailed risk‑benefit discussion. Finally, keep a written log of every symptom, supplement, and medication change; this record empowers you and your oncology team to make data‑driven adjustments that keep you on therapy for the full intended duration while preserving quality of life.
Michelle Abbott
September 1, 2025 AT 06:13From a pharmacokinetic perspective, anastrozole exhibits a high hepatic first‑pass metabolism via CYP3A4, which predisposes it to numerous drug‑drug interaction vectors that are often underappreciated in routine oncology practice; clinicians must therefore conduct a meticulous reconciliation of concomitant agents, especially statins, antifungals, and selective serotonin reuptake inhibitors, to avoid unpredictable plasma concentrations that could exacerbate musculoskeletal toxicity. Moreover, the ligand‑induced conformational shift in the estrogen receptor-though ostensibly mitigated by peripheral aromatase blockade-does not fully abrogate estrogenic signaling in non‑target tissues, thereby perpetuating a residual risk of cardiovascular events that is poorly captured in short‑term trial endpoints. The reliance on surrogate biomarkers such as disease‑free survival, while expedient, masks the long‑term cost‑benefit calculus that includes osteoporosis‑related morbidity and the societal burden of increased fracture incidence, a factor that health‑economics models frequently discount. In light of these multifactorial considerations, a blanket recommendation for extended anastrozole therapy without stratified risk assessment is scientifically tenuous and ethically questionable.